연구/산학
PKNU Research 1000
| Jung Sung-Chul | Presents a plan to improve electrolyte performance of all-solid-state batteries | |||
| 작성자 | 대외협력과 | 작성일 | 2024-01-12 |
| 조회수 | 615 | ||
| Jung Sung-Chul | Presents a plan to improve electrolyte performance of all-solid-state batteries | |||||
 |
대외협력과 |  |
2024-01-12 |  |
615 |
PKNU research team presents a plan to improve electrolyte performance of all-solid-state batteries
- research results of prof. Jung Sung-Chul's team published in the international journal of the Royal society of chemistry
- the disorder of anions increases the stability of the solid electrolyte
Pukyong National University (President Jang Young-Soo) announced that a research team led by professor Jung Sung-Chul (department of physics) proposed a method to improve the performance of Li6PS5Cl, a sulfide-based solid electrolyte used in solid-state batteries.
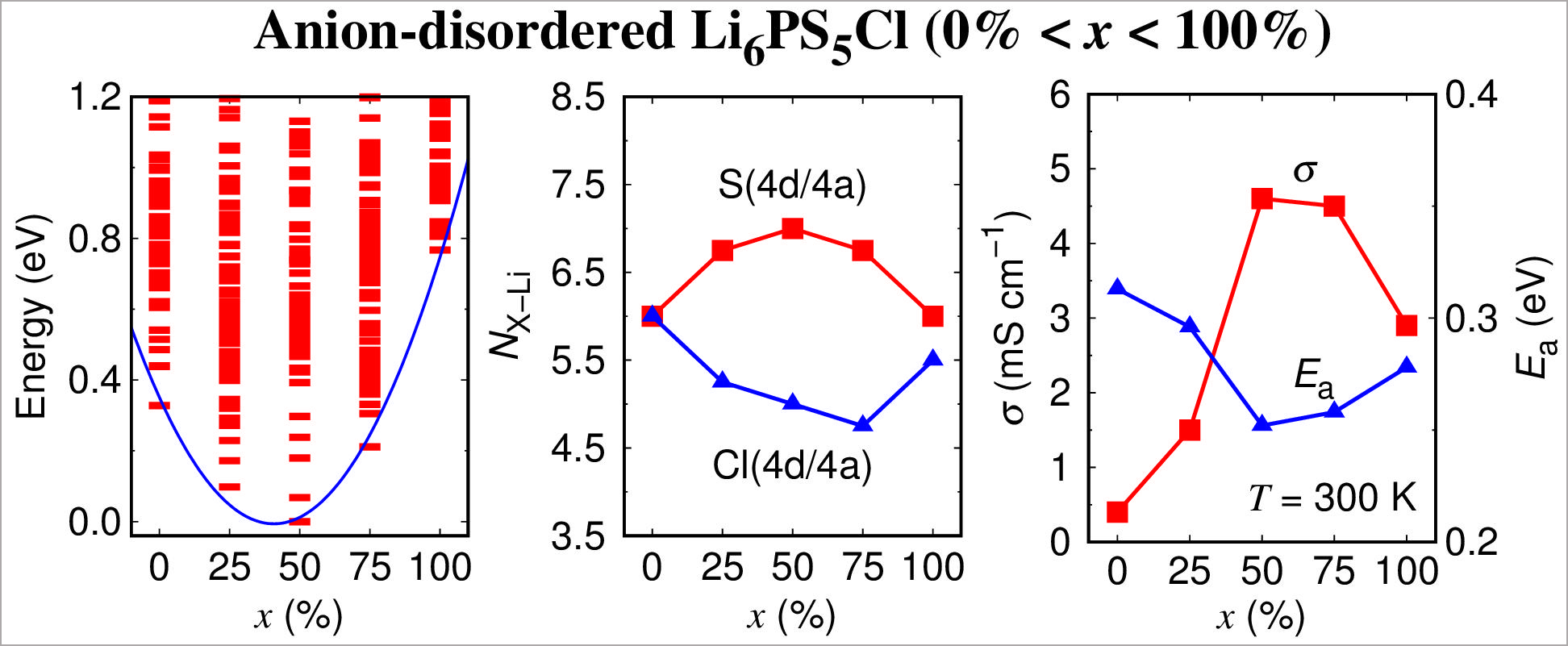
In an ab initio calculation study conducted by professor Jung Sung-Chul with phd student Jeon Tae-Gon and master's researcher Cha Kyeong-Ho in the same department, he succeeded in quantitatively identifying the effect of the disordered arrangement of S and Cl anions in Li6PS5Cl, a solid electrolyte, on the physical properties of Li6PS5Cl.
All-solid-state batteries are receiving a lot of attention because they can greatly increase the stability and energy density of the battery by replacing the flammable liquid electrolyte with a non-flammable solid electrolyte. Li6PS5Cl, based on the argyrodite structure, has excellent ionic conductivity, and its raw materials, Li2S, LiCl, and P2S5, are economical and easy to synthesize, so many studies are being pursued.
In this study, professor Jung's team found that Li6PS5Cl is highly stabilized when disorder occurs compared to when there is no anion disorder, and the stabilized Li6PS5Cl shows high ionic conductivity (up to 4.6 mS cm 1).
Additionally, the research team found that when anion disorder occurs, some of the Li ions move from around Cl to close to S, and that this rearrangement of Li contributes decisively to improving the stability and increasing conductivity of Li6PS5Cl.
Professor Jung Sung-Chul said, "I believe that this study suggests that precisely controlling the ionic disorder of the solid electrolyte is another way to improve the electrochemical performance of not only Li6PS5Cl but also other solid electrolytes."
The study was conducted through support from the mid-career researcher program supported by the Ministry of science and ICT and the LAMP project hosted by the Ministry of education, and recently published in <Journal of materials chemistry A> (IF=11.9), a renowned international journal in the fields of chemistry, physics, and materials published by the Royal society of chemistry in the UK.

△ The researchers (from left, Jung Sung-Chul, Jeon Tae-Gon, and Cha Kyeong-Ho).