연구/산학
PKNU Research 1000
| Eom Woo-ram | Develop New Cancer Treatment Technology Using ‘Nano-Bubbles’ | |||
| 작성자 | 대외협력과 | 작성일 | 2024-10-25 |
| 조회수 | 124 | ||
| Eom Woo-ram | Develop New Cancer Treatment Technology Using ‘Nano-Bubbles’ | |||||
 |
대외협력과 |  |
2024-10-25 |  |
124 |
Pukyong National University·Tech University of Korea·Sungkyunkwan University, Develop New Cancer Treatment Technology Using ‘Nano-Bubbles’
-Nano-Bubbles Responsive to Ultrasound… Destroying Cancer Cell Nuclei to Treat Metastatic Cancer
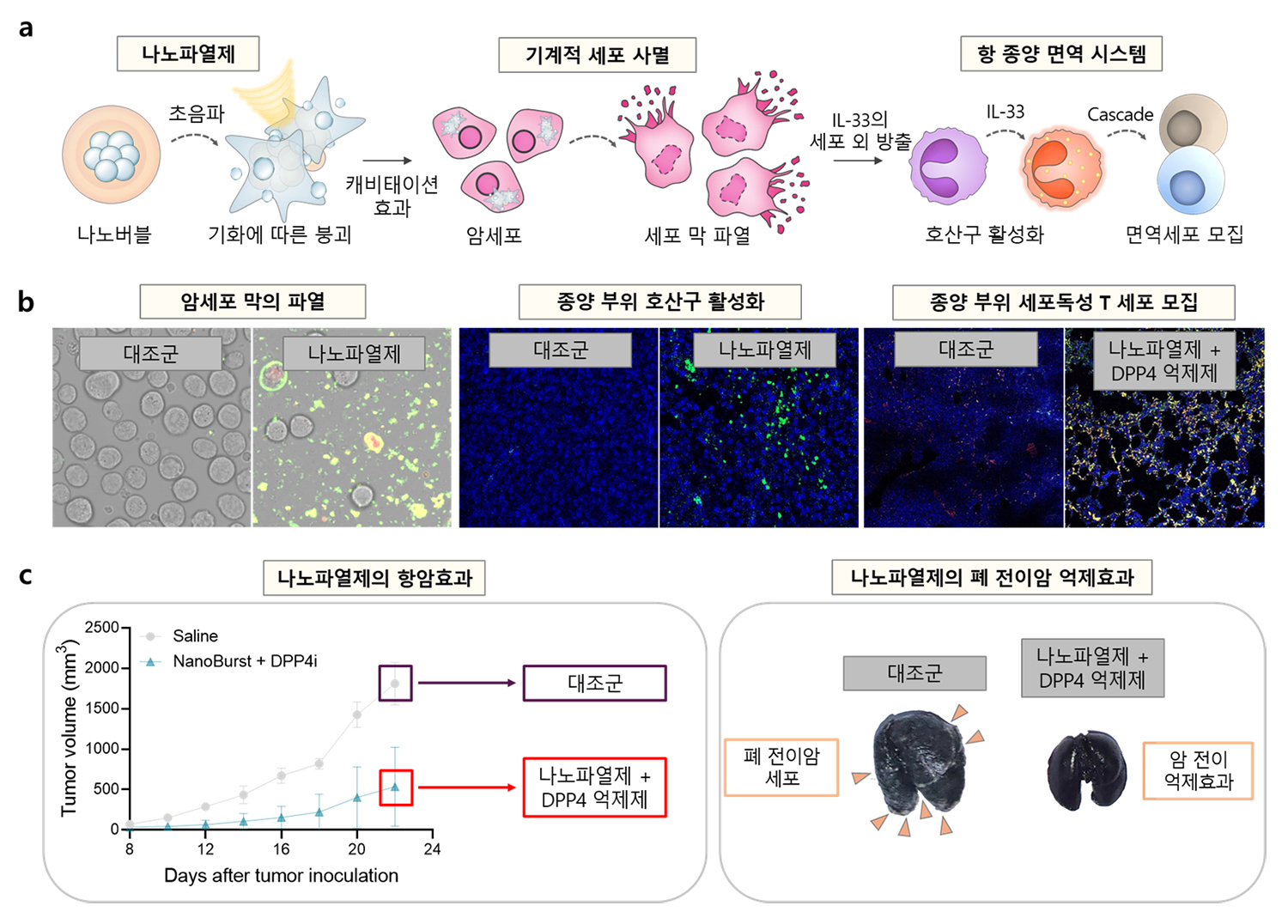
Professor Eom Woo-ram from the Department of Bioengineering at Pukyong National University, Professor Yoo Dong-gil from Tech University of Korea, and Professor Park Jae-hyung from Sungkyunkwan University have successfully developed a new type of mechanical cell death method(caviptosis) that can dramatically enhance the efficacy of cancer immunotherapy, marking the first development of its kind in the world.
Cancer immunotherapy is a method of treating cancer by utilizing the body's immune function, and it is gaining attention for its lower side effects and superior treatment effects compared to traditional chemotherapy. In particular, immune checkpoint inhibitors, which are a representative method of immunotherapy capable of normalizing immune responses disrupted by cancer cells, are being actively applied clinically to treat various types of cancer.
However, recent studies have shown that when there is a deficiency in the number of cytotoxic T cells present in the tumor microenvironment, the effectiveness of immune checkpoint inhibitor therapy significantly decreases. Consequently, there has been a continuous demand for new technologies that can attract external immune cells to the tumor site to enhance the efficacy of cancer immunotherapy and expand treatment benefits to a larger patient population.
Professor Eom Woo-ram's research team focused on the role of eosinophils, a type of white blood cell. Although eosinophils are generally known to have a negative impact on cancer treatment, they can be stimulated by interleukin-33 (IL-33), which resides inside the cell nucleus, to attract immune cells into the tumor and exhibit strong anti-cancer effects. However, IL-33 is tightly bound within the cell nucleus, making treatment options utilizing eosinophils and IL-33 extremely limited.
Professor Eom Woo-ram's research team successfully developed a mechanical cell death method (cavitopsis) that can rupture cells when exposed to ultrasound by utilizing nano-sized bubbles capable of penetrating into the cells.
The nano-explosive agent made from nano-bubbles responsive to ultrasound ruptures the nuclei of cancer cells, releasing IL-33, which is hardly released in its natural state, into the extracellular space. This IL-33 stimulates eosinophils to attract immune cells into the tumor, elucidating its anti-cancer effect. The research team validated the therapeutic efficacy of the nano-explosive agent in a real lung metastatic cancer mouse model, confirming a dramatic enhancement in the anti-cancer efficacy of existing immune checkpoint inhibitors.
The principal investigator, Professor Eom Woo-ram, stated, “Currently, cancer immunotherapy is very expensive, costing millions of won, and has limitations as it is effective only for a subset of patients. However, I expect that this research will dramatically enhance the therapeutic efficacy of cancer immunotherapy, increasing the potential for treating metastatic cancers that have been difficult to treat with existing therapies.”
The research team conducted this study with the support of the National Research Foundation of Korea’s Individual Basic Research and National New Drug Development projects. The research results were published in the international journal <Advanced Functional Materials>, with researcher Song Ye-ri (Sungkyunkwan University) and Professor Eom Woo-ram as first authors and Professors Eom Woo-ram and Park Jae-hyung as corresponding authors.
Meanwhile, Professor Eom Woo-ram's research team is conducting follow-up research to develop cancer and obesity treatment methods utilizing nanotechnology that can regulate cellular functions.